Abstract
Background: Patients with relapsed indolent NHL (iNHL) have limited standard treatment options. Lenalidomide combined with rituximab (R 2) has shown complimentary clinical activity and is a tolerable regimen in both untreated and relapsed or refractory (R/R) patients with iNHL (RELEVANCE : N Engl J Med 2018;379:934 and AUGMENT: J Clin Oncol. 2019;37:1188).
Methods: MAGNIFY is a multicenter, phase 3b trial in patients with R/R follicular lymphoma (FL) grades 1-3b, transformed FL (tFL), marginal zone lymphoma (MZL), or mantle cell lymphoma (MCL; NCT01996865) exploring optimal lenalidomide duration. In the induction phase, lenalidomide 20 mg PO on days 1-21 of a 28-day cycle + rituximab IV at 375 mg/m 2/week cycle 1 and then every 8 weeks starting with cycle 3 (R 2) are administered for 12 cycles. Patients with stable disease, partial response, or complete response/complete response unconfirmed (CR/CRu) were randomized 1:1 to R 2 vs rituximab maintenance for 18 months. Data presented here are the complete analysis from the induction phase in efficacy-evaluable patients with FL grades 1-3a or MZL (FL grade 3b, tFL, and MCL not included). The focus of this interim analysis was overall response rate (ORR) by 1999 IWG criteria in the induction intention-to-treat population.
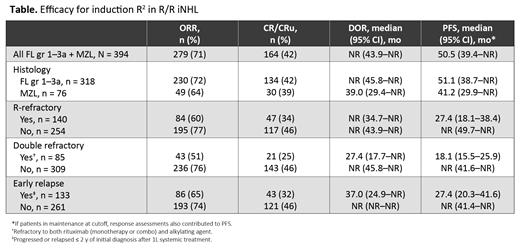
Results: As of March 5, 2021, 394 patients (318 [81%] FL gr1-3a; 76 [19%] MZL) were enrolled. The median follow-up was 40.6 mo (range, 0.6-79.6). Median age was 66 y (range, 35-91), 328 (83%) had stage III/IV disease, with a median of 2 prior therapies (94% prior rituximab-containing). ORR was 71% (n = 279) with 42% (n = 164) CR/CRu (Table). All patients have completed R 2 induction (n = 232, 59%) or discontinued study treatment (n = 162, 41%). 141 patients (36%) prematurely discontinued both lenalidomide and rituximab, primarily due to adverse events (AEs) (n = 54, 14%) or progressive disease (n = 42, 11%). The majority of patients who have completed induction have been randomized and entered maintenance (n = 217). Median duration of response in the induction period was not reached (95% CI, 43.9 mo-NR), and median progression-free survival in the induction safety population (n = 393) was 50.5 mo (95% CI, 39.5-NR). Efficacy results are reported in the table by histology subgroups (FL vs MZL), and rituximab-refractory, double-refractory, and early relapse statuses. Most common all-grade AEs were 47% fatigue, 43% neutropenia, 37% diarrhea, 30% nausea, and 30% constipation. Grade 3/4 AEs occurring in ≥ 5% of patients included 37% neutropenia (10 patients [3%] had febrile neutropenia), 8% leukopenia, 6% thrombocytopenia, 5% anemia, and 5% fatigue.
Conclusions: These data represent complete analysis of all patients in the induction phase of MAGNIFY which continue to support that R 2 is active with a tolerable safety profile in patients with R/R FL grade 1-3a and MZL, including rituximab-refractory, double-refractory, and early relapse patients.
Lansigan: Celgene/BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees. Andorsky: Abbvie: Consultancy, Research Funding; Celgene/BMS: Consultancy, Research Funding; Epizyme: Research Funding. Coleman: immunomedics: Current equity holder in publicly-traded company, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company; Abbvie: Research Funding; Bristol Myers: Research Funding; Celgene: Research Funding; Genentech: Research Funding; Gilead: Research Funding; BeiGene: Research Funding; Innocare: Research Funding; Merck: Research Funding; Pfizer: Research Funding; Roche: Research Funding. Yacoub: Dynavex: Current equity holder in publicly-traded company; Cara: Current equity holder in publicly-traded company; Ardelyx: Current equity holder in publicly-traded company; Agios: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ACCELERON PHARMA: Membership on an entity's Board of Directors or advisory committees; CTI Biopharma: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Consultancy, Honoraria, Speakers Bureau; Seattle Genetics: Honoraria, Speakers Bureau; Hylapharm: Current equity holder in publicly-traded company. Melear: TG Therapeutics: Speakers Bureau; Astrazeneca: Speakers Bureau; Janssen: Speakers Bureau. Fanning: Sanofi: Speakers Bureau; Genmab: Membership on an entity's Board of Directors or advisory committees; TG Pharma: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Genentech: Membership on an entity's Board of Directors or advisory committees; Takeda: Speakers Bureau; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; BMS: Speakers Bureau. Kolibaba: TG Therapeutics: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company; Atara Biotechm: Consultancy; McKesson Specialty Health: Consultancy; Sunitomo Dainippon Pharma: Consultancy; Tolero Pharma: Consultancy, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company). Nowakowski: Incyte: Consultancy; Kymera Therapeutics: Consultancy; TG Therapeutics: Consultancy; Blueprint Medicines: Consultancy; Nanostrings: Research Funding; Curis: Consultancy; Selvita: Consultancy; Zai Labolatory: Consultancy; Daiichi Sankyo: Consultancy; Bantham Pharmaceutical: Consultancy; Karyopharm Therapeutics: Consultancy; Ryvu Therapeutics: Consultancy; Genentech: Consultancy, Research Funding; Kyte Pharma: Consultancy; Roche: Consultancy, Research Funding; Celgene/Bristol Myers Squibb: Consultancy, Research Funding; MorphoSys: Consultancy. Gharibo: BMS: Current Employment, Current equity holder in publicly-traded company, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Ahn: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company; ADC therapeutics: Current equity holder in publicly-traded company. Li: BMS: Current Employment, Current equity holder in publicly-traded company, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Research Funding. Sharman: BeiGene: Consultancy; TG Therapeutics: Consultancy; Lilly: Consultancy; Centessa: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics LLC, an AbbVie Company: Consultancy; AstraZeneca: Consultancy; BMS: Consultancy; AbbVie: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal